BIS Certification for Single Volume Pipettes IS 1117: 2018

Product Description
Single Volume Pipettes are essential laboratory tools designed for precise measurement and transfer of fixed liquid volumes. Made from high-quality, transparent glass, these pipettes are meticulously calibrated to ensure accurate and consistent results in various scientific experiments and procedures. Their sleek, narrow design allows for easy handling and reduces the margin of error, making them indispensable in fields requiring exact liquid measurements.
These pipettes are available in various sizes to accommodate different volume needs, offering flexibility in both research and routine laboratory tasks. Durable and resistant to chemical reactions, Single Volume Pipettes are built to withstand repeated use while maintaining accuracy. Their clear graduations and markings provide easy readability, ensuring that each measurement is as precise as possible, contributing to reliable and reproducible laboratory outcomes.
Introduction
BIS Certification for Single Volume Pipettes is a mark of quality and reliability, ensuring that these laboratory instruments meet the stringent standards set by the Bureau of Indian Standards (BIS). The certification process involves rigorous testing to verify that the pipettes are made from high-grade materials, deliver precise measurements, and withstand regular use without degradation. By obtaining BIS Certification, manufacturers can demonstrate their commitment to producing pipettes that are safe, accurate, and dependable for critical laboratory work.
Why is BIS Certification Necessary for Single Volume Pipettes?
BIS Certification is essential for Single Volume Pipettes to ensure they meet stringent safety and quality standards set by the Bureau of Indian Standards (BIS). According to the Laboratory Glassware (Quality Control) Order, 2023, issued by the Ministry of Commerce and Industry, it is mandatory for these pipettes to obtain BIS certification and carry the ISI mark. Compliance with this regulation guarantees that the pipettes are reliable and precise for laboratory use. Manufacturers who fail to meet these requirements risk legal penalties, including fines, imprisonment, or both, and their products may be sealed by BIS if not properly labeled with the ISI mark. This certification is crucial for maintaining the integrity and safety of laboratory practices.
Also Read
BIS QCO for Single Volume PipettesOverview of Indian Standard IS 1117:2018
for Single Volume Pipettes
The Indian Standard IS 1117:2018 specifies the requirements and guidelines for the design, construction, and performance of Single Volume Pipettes used in laboratory settings. This standard ensures that pipettes are made from high-quality, durable glass that can withstand regular use and maintain accuracy over time. It covers key aspects such as dimensional accuracy, calibration, chemical resistance, and the clarity of graduations and markings. By adhering to IS 1117:2018, manufacturers ensure that their Single Volume Pipettes deliver precise and reliable measurements, essential for maintaining consistency and accuracy in scientific research and laboratory work.
Testing Required for BIS Certification
To obtain BIS
Certification (ISI Mark) for Single Volume Pipettes, the product must
undergo rigorous testing as per IS 1117:2018. The tests ensure that the
cylinders meet the necessary standards in terms of performance and safety. The
key tests include:
● Maximum Permissible Errors: Ensures
pipettes meet accuracy standards.
● Material: Verifies high-quality, durable
glass is used.
● Dimensions: Confirms pipette size and
capacity are precise.
● Delivery Time: Checks that liquid
delivery is within the specified time.
● Graduation Line: Ensures clear, accurate
markings for precise measurements.
Process of BIS Certification for Single Volume Pipettes
Obtaining BIS certification for Single Volume Pipettes involves several steps. The process is designed to ensure that all products meet the required standards before they reach consumers. Here’s a step-by-step guide:
Step 1. Application Submission: Submit an application to BIS with the required fees, including product details, manufacturing process, and quality control measures.
Step 2. Product Testing: The Single
Volume Pipettes is tested in a BIS-recognized lab to ensure compliance with IS 1117:2018 standards.
Step 3. Factory Inspection: BIS
officials inspect the manufacturing facility to verify adherence to standards,
including raw materials, processes, and quality control.
Step 4. Evaluation of Test Reports: BIS
evaluates the lab test results. If the product meets all standards, it’s
approved for certification.
Step 5. Grant of BIS License: Upon
meeting all requirements, BIS grants the license, allowing the use of the ISI
mark on the product.
Step 6. Marking and Labeling: Ensure
that all certified products display the ISI mark, license number, and relevant
information.
Documents Required for BIS Certification
To apply for BIS certification, manufacturers need to submit the following documents:
● Application form
● Manufacturing process details
● Quality control plan
● Test reports from BIS-approved laboratories
● Factory layout and equipment details
● Proof of business registration
● Product specifications and technical details
● Declaration of conformity to Indian standards
Additionally, manufacturers may be required to provide proof of compliance with environmental and safety regulations, depending on the specific type of product being certified.
BIS ISI Mark Certification Costing And Timeline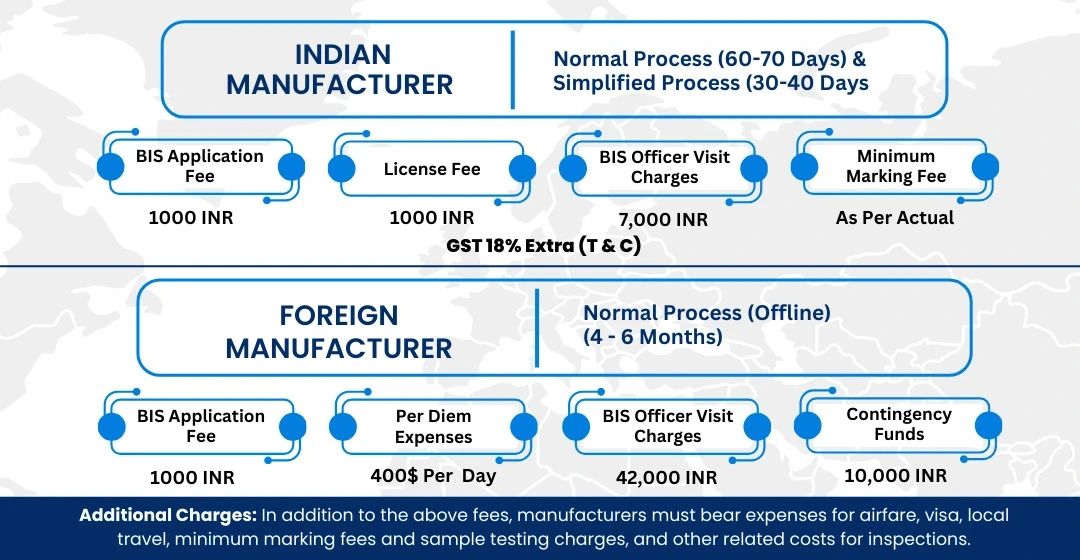
To Know The Process in Detail, Please Visit:
Under BIS Registration Products ISI and CRS
Benefits of BIS Certification
Obtaining BIS Certification for laboratory glassware offers several benefits:
- Compliance with Regulatory Requirements: It helps manufacturers comply with Quality Control Orders (QCO) and other regulatory mandates in India.
- Enhanced Market Access: Certified products are more likely to be accepted in both domestic and international markets (import/export).
- Consumer Trust: The ISI Mark instills confidence in consumers about the product's quality, safety, and reliability.
- Competitive Advantage: BIS-certified products stand out in the market, often leading to better sales and customer loyalty.
- Online BIS Portal Access: Facilitates easy access to certification-related information and procedures, streamlining the process for manufacturers.
Conclusion: How EVTL India Can Help Manufacturers Obtain BIS Certification
EVTL
India is a leading consultancy firm that assists manufacturers of
laboratory glassware, including Single Volume Pipettes, in obtaining BIS
Certification (ISI Mark). The process involves several steps, including
application submission, product testing, and compliance verification. EVTL India
offers end-to-end support, ensuring that manufacturers meet all the
requirements of IS 1117:2018 efficiently.
With expertise
in navigating the complexities of BIS Certification, including online BIS
portal management, documentation, and regulatory fees, EVTL India ensures a
smooth and successful certification process. By choosing EVTL India,
manufacturers can enhance their product's marketability, both within India and
internationally, securing their place in the competitive laboratory glassware
industry.
Whether you're
dealing with import or export, or looking to comply with the latest QCO Orders,
EVTL India is your partner in achieving BIS Certification.
Free Call Back
Latest News & Update
📅 BIS Critical Component List (CCL) Updates for Solar PV Modules
🕒 BIS Fee Concessions for MSMEs and Startups | EVTL India
📅 Guidelines for Implementation of Essential Requirements for Security of CCTV
🕒 Machinery and Electrical Equipment Safety (Omnibus Technical Regulation) Order, 2024
🕒 Extension of Timeline for Filing Annual Returns by Battery Producers
📅 Extension of Timeline for Filing Quarterly and Annual Returns for E-Waste
🕒 Extension of Concurrent Running Period for IS 302-1: 2008 and IS 302 (Part 1): 2024